
Technology - www.cabenda.com/technology.html

Technology - www.cabenda.com/technology.html
Recent advances in immunohistochemistry, robotic microscopy and computing mean that it is now feasible to scan entire tumour cryosections and map drugs and biological markers relative to tumour vasculature (1-4). Using an automated epifluorescence microscope and a capillary-based immunostaining system allows us to provide higher throughput tumour mapping of multiple markers.
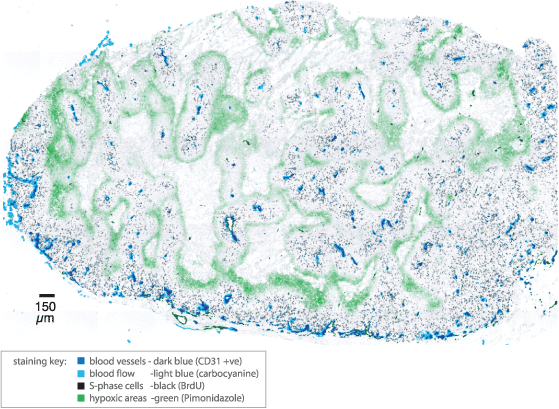
Evaluating drug penetration
Simultaneous mapping of radiolabelled or immunodetected drugs and tumour vasculature allows for evaluation of drug penetration as a function of distance from vasculature.
Assessing microregional effect of drugs
Mapping other markers such as markers of blood flow, proliferation (e.g. BrdUrd, Ki67, PCNA), apoptosis (e.g. TUNEL, caspase 3) and DNA damage (e.g. gamma-H2AX) can be used to map the effect of drugs via their effect on cells in tumours relative vasculature. Allowing for rapid assessment of the microregional distribution and effect of anticancer agents.
Limitations of tumour mapping for evaluation drug penetration
While tumour mapping represents a relatively new and unexplored area of research, numerous limitations exist in terms of employing it in assessing drug penetration. With regards to direct imaging, radiolabeled drugs are not always available and are generally expensive. Autoradiography-based experiments are labour intensive and getting results can take months. In addition, the potential for presence of radiolabelled drug metabolites means that data will be often be inconclusive. Effect-based assays are prone to changes in intrinsic sensitivity of cells to drugs with proliferation status, oxygenation etc. In addition host toxicity from the drug may effect proliferation and even apoptosis in a tumour independent of drug distribution via reduction in oxygen and nutrients in the blood. These limitations call out for in vitro assays which allow the individual factors to be studied in isolation from one another.
We provide a range of in vitro assay solutions that can be used in a semi-high throughput mode to expedite the identification of lead molecules
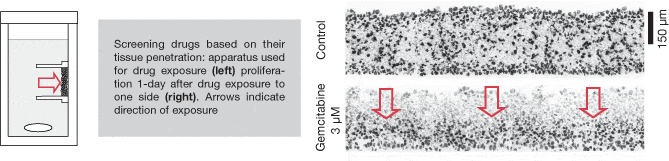
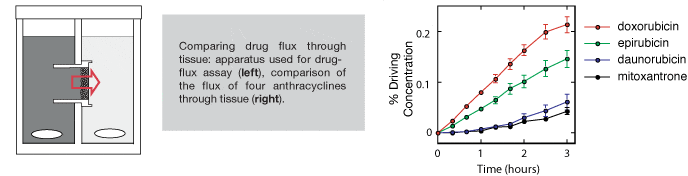
Multilayered cell culture (MCC)
A variation on multicellular spheroidal cell culture in which discs of tissue are grown from tumour cell lines (5, 6). A variety of tumour cell lines (HCT-116, MCF7, SiHa) can be grown using this technique. Cells are added to collagen coated tissue culture inserts and allowed to attach overnight to form a confluent layer. They are then immersed in stirred media and grown over 2-5 days to form discs of tissue typically 15-30 layers in thickness.
References:
1. Ljungkvist AS, Bussink J, Rijken PF, Kaanders JH, van der Kogel AJ, and Denekamp J. Vascular architecture, hypoxia, and proliferation in first-generation xenografts of human head-and-neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys 2002;54:215-228.
2. Huxham LA, Kyle AH, Baker JH, McNicol KL and Minchinton AI. Tirapazamine causes vascular dysfunction in HCT-116 tumour xenografts. Radiother Oncol 2006;78:138-145.
3. Huxham LA, Kyle AH, Baker JH, Nykilchuk LK and Minchinton AI. Microregional effects of gemcitabine in HCT-116 xenografts. Cancer Res 2004;64:6537-6541.
4. Kyle AH, Huxham LA, Baker JH, Burston HE and Minchinton AI. Tumor distribution of bromodeoxyuridine-labeled cells is strongly dose dependent. Cancer Res 2003;63:5707-5711.
5. Cowan DSM, Hicks KO and Wilson WR. Multicellular membranes as an in vitro model for extravascular diffusion in tumours. Br. J. Cancer 1996;74:S28-S31.
6. Minchinton AI, Wendt KR, Clow KA and Fryer KH. Multilayers of cells growing on a permeable support: An in vitro tumour model. Acta Oncologica 1997;36:13-16.
7. Kyle A Modelling extravascular drug penetration using multilayered cell culture. Department of Physics, Vol. M.Sc., pp. 133. Vancouver: Univeristy of British Columbia, 1999.
8. Hicks KO, Fleming Y, Siim BG, Koch CJ and Wilson WR. Extravascular diffusion of tirapazamine: effect of metabolic consumption assessed using the multicellular layer model. Int J Radiat Oncol Biol Phys 1998;42:641-649.
9. Kyle AH and Minchinton AI. Measurement of delivery and metabolism of tirapazamine to tumour tissue using the multilayered cell culture model. Cancer Chemother Pharmacol 1999;43:213-220.
10. Phillips RM, Loadman PM and Cronin BP. Evaluation of a novel in vitro assay for assessing drug penetration into avascular regions of tumours. Br J Cancer 1998;77:2112-2119.
11. Hicks KO, Ohms SJ, van Zijl PL, Denny WA, Hunter PJ, and Wilson WR. An experimental and mathematical model for the extravascular transport of a DNA intercalator in tumours. British Journal of Cancer 1997;76:894-903.
12. Tunggal JK, Melo T, Ballinger JR and Tannock IF. The influence of expression of P-glycoprotein on the penetration of anticancer drugs through multicellular layers. Int J Cancer 2000;86:101-107.