
Tumour Microenvironment - www.cabenda.com/tm.html

Tumour Microenvironment - www.cabenda.com/tm.html
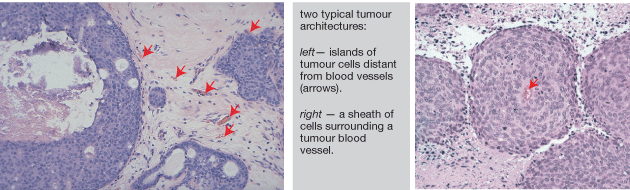
In contrast to most normal tissues, with relatively high microvessel densities, the extravascular compartment of solid tumors often poses a significant barrier to the penetration of molecules supplied by the blood. Deregulated proliferation of tumor cells causes increased separation of blood vessels (1, 2) and unstable perfusion (3-6), which in turn leads to a reduction in the ability of molecules supplied from the blood to reach all cells within the tissue (7, 8). Tumor cells can be located up to 15-20 cells away from the nearest blood vessel (more in some cases) while in most normal tissue cells are within a few cell layers of a vessel.
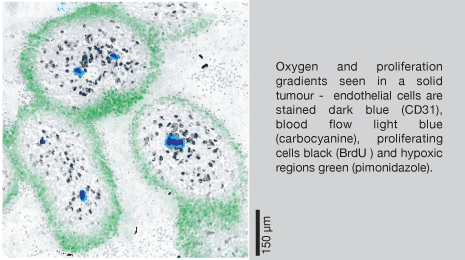
biological gradients
Sparse or aberrant vasculature and deregulated tumour cell proliferation combine to create gradients in biological molecules such as oxygen, glucose and build up of waste products such as lactic acid and reduction in proliferation with distance away from vessels. All this creates a wide range of environmental conditions which, when linked with sub-therapeutic drug exposures in distant regions and genomic instability, can be responsible for producing aggressive phenotypes seen in later stages of tumour progression. In fact, the gradients in physiological molecules and therapeutic agents that are observed with distance from vasculature in solid tumours could be considered the ideal system for production and selection of cell variants that will ultimately lead to tumour progression.
drug penetration
Little is known of the ability of many of the most commonly used anticancer drugs to distribute within solid tumors (9). Measurement of gross tumor drug accumulation is a routine component of new drug development but tells little about extravascular drug distribution, especially in the case of drugs that are highly reactive in tissue.

References:
1. Rubin P and Casarett G. Microcirculation of tumors - Part I: Anatomy, Function and necrosis. Clin. Radiol. 1966;17:220-229.
2. Thomlinson RH and Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br. J. Cancer 1955;9:539-549.
3. Brown JM. Evidence for acutely hypoxic cells in mouse tumours and a possible mechanism of reoxygenation. Br. J. Radiol. 1979;52:650-656.
4. Durand RE and LePard NE. Contribution of transient blood flow to tumour hypoxia in mice. Acta Oncologica 1995;34:317-323.
5. Chaplin DJ, Durand RE and Olive PL. Acute hypoxia in tumour: Implications for modifiers of radiation effects. In. J. Radiat. Oncol. Biol. Phys. 1986;12:1279-1282.
6. Minchinton AI, Durand RE and Chaplin DJ. Intermittent blood flow in the KHT sarcoma - flow cytometry studies using Hoechst 33342. Br. J. Cancer 1990;62:195-200.
7. Jain RK. Transport of molecules in the tumor interstitium: a review. Cancer Research 1987;47:3039-3051.
8. Jain RK. Delivery of molecular and cellular medicine to solid tumors. Adv Drug Deliv Rev 1997;26:71-90.
9. Tannock IF, Lee CM, Tunggal JK, Cowan DS and Egorin MJ. Limited penetration of anticancer drugs through tumor tissue: a potential cause of resistance of solid tumors to chemotherapy. Clin Cancer Res 2002;8:878-884.